It is made up of atoms and molecules which contain mass.
The three units of mass:
gram g
kilogram kg
milligram mg
Example:
A small penny coin has a mass of 2.5g

However, the mass of the M87 black hole can be as much as 3.580056 × 1031 kg!

For a grain of sand, it is estimated to have a mass of 2.5mg.

Matter can be seperated into two categories: "Mixture" and "Pure Substance".
Mixture
- Made out of substances that CANNOT be united during any chemical processes
- Have more than one set of properties
- Example: salt water, alcohol
- Can be classified as "Homogeneous" or "Heterogeneous"
Homogeneous

↑ Sugar added in a glass of water
~Different components are NOT visible to naked eye after being mixed
~ The components are spreaded uniformly throughout mixing
~ They do not undergo any reactions
~ Examples: sugar in coffee, milk in tea
Heterogeneous

↑ Oil floating on top of a glass of water
~ Different components can be visibly seen after mixed together
~ Components seem to have very different properties so they don't blend
~ Physical appearence of components will not be changed after mixed
~ Examples: oil with water, noodles with pork, sand in water
Pure Substance
- Cannot be physcially or chemically seperated
- Have the same taste, color, composition, and texture between the substances
- Have only one set of properties
- Examples: Gold, oxygen gas
- Can be classified into an "Element" or a "Compound"
Element
~ Composed of atoms
~ The simplest form of matter
~ Cannot be decomposed or break down
~ There are 115 known elements
~ Example: hydrogen, nitrogen, silver
~ Can classified into "Metal", "Metalloid" and "Non-metal"
Metals

↑ A testube with mercury
‧Metal elements are great conductors of heat and electricity
‧High melting points
‧Solid at room temperature
‧Example: iron, copper, silver
Metalloid

↑ Boron, a metalloid
‧They have both metal and non-metal properties
‧They are like non-metals when they "meet with" metals
‧They are like metals when the "meet with" metals
‧Example: boron, silicon
Non-metal

↑ Neon is an example of a non-metal
‧Heat and electricity isulators
‧Gain electrons easily
‧Example: neon, chlorine
Compound
~ They are made up of two or more elements
~ Compounds are combined chemically
~ Molecules such as oxygen gas should composed of 2 atoms
~ Example: Chlorine gas, sulfur dioxide
Ionic-acid
‧Has a pH <7
‧Corrosive
‧Taste sour
‧Always have a hydrogen atom
‧Example: acetic acid, hydrochloric acid
Ionic-Base
‧Has a pH >7
‧Corrosive
‧Taste bitter
‧Always have a hydroxide atom
‧Example: ammonia, sodium hydroxide
Ionic-Salt
‧The mixture of an acid and a base (neutralization)
‧Always have a salt product + H2O
‧Example: HCl + NaOH → NaCl + H2O
Covalent-Organic compound
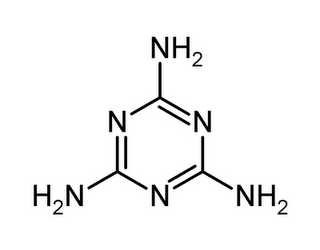
↑ The structure formula of Melamine
‧A compound with a carbon atom
‧Example: Aspartic acid, Hexafluoropropylene
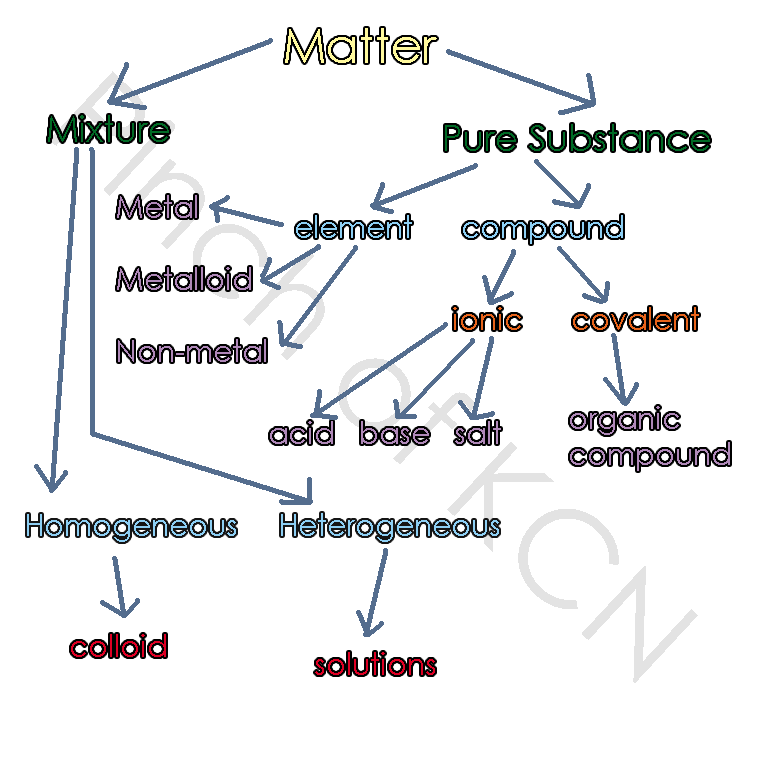
The diagram of Matter and it's sub-categories:

No comments:
Post a Comment