
Helllo ya' all. I feel like we're contradicting ourselves lately. Wait, why is my nose growing???? When we say that was our last blog post of the year, it wasn't. When we say that was going to be our last joke of the year, we didn't mean so. Hopefully these deceiving words of ours didn't hesitate you too much from continuing reading our blog, our very last blog post. Anyways, bear with me for one last time, I got a little treat for you all, an internet (not cookie) treat of course, like usual.
[ DON'T SCROLL DOWN JUST YET, I WARN YOU]
So today, our last day together, we'll be looking over the topics called 'Alcyclics and Aromatics.'
Alcyclics, also know as cyclic hydrocarbons is hydrocarbon chain which connect in a circle formation. The prefix "CYCLO-" is used for naming molecules in such formation. The general formula for alcyclics is C
NH2N. Yes, it's simple as that!
Let's show it in a example:

Say in diagram #1, top right, there's 3 carbons, which means it's a propane. And since it's an alcyclics, it's then cyclo + propane, which makes it cyclopropane. Na da, easy piesy right?!
As for alkenes and alkynes, simply make the first and second carbon be where the double and/or triple bond situate(s). Therefore, there's no need to indicate the "1" in front of the molecule then for indication purposes.
For branched cycloaka/e/ynes molecules, naming is just the same as we have done before. Let's recap with a slightly newer example:

<--- 1) As for naming, first count the number for the longest carbon chain. In this case it's 6, which means it's a hexane as there's no double nor there's a triple bond.
2) Then you see, it's a cyclic, now add the cyclo- onto it. Now it makes a cyclohexane.
3) Now you look again, you notice there's a CH3 sticking out from one of he carbon. You should treat it the same as we always do. CH3 is also methane. As a side chain, add prefix "methyl-" to cyclohexane, which makes methylcyclohexane. DONE!
Moving on, we got Aromatics. From the word itself, you can smell the aroma already. LOL. Aromatic molecule is a molecule that contains one or more benzene rings. Benzene is C6H6, which holds a structure like the following,
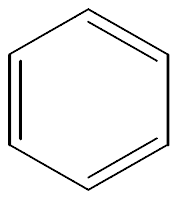
Benzene has the potential to become a parent chain or a side chain. For a side chain, the prefix "phenyl-" is added. For a parent chain, the word "-benzene" is attached as a suffix.
Example time again:

propyl- + -benzene = propylbenzene

2-phenyl +butane = 2-phenylbutane
You may ask: What's up with that circle stuff in the benzene ring, that shows that electrons can move around the ring. Nothing big. To understand it easier, we suggest you to ignore the meaning of it as it will not affect your naming, aka your grades. Jokes!
Since we're saying goodbye to all of our wonderful blog supporters, we are willing to reveal our identities... just slightly...because we all know the World Wide Web is never too trustworthy. Anyways, if you haven't noticed already, our blog is not written by a single person, in fact, it's done by a committee of writers. There are four of us. Just yesterday, a Disney animated cartoon called Recess struck me and I realized wow those four characters do have some parallels with us.
Here is C.L., best represented by Gus Griswald. He is a magnificent storyteller. One of his greatest creation must be the
Mole King. And needless to say, he is a super nerdy guy:) Let me show you a head-shot of him:

![]()

**Maybe a bit taller than Gus...**
And then, we gotta mention our talented computer geek K.L., she's so good at anything involving computer that I bound to call her my computer master. Her greatest achievement definitely has to be her epic
Mole King Game. She is best depicted by Gretchen Grundler. Without further ado, let's find out what she looks like,


**Minus the chipmunk teeth and those lovely 'Wizard of Oz' pigtails...looks just right :)**
Oh dear, here we come, our "most dedicated"...ahem...
NOT AT ALL...member of our blog committee. That would be our best description of him. His name is Mikey C. Like in the animated TV show, he best resembles Mikey Blumberg. Our nickname for him is Big Mikey because he loves to eat Big Mac...get it?! LOL.
Mikey before Chem class: Mikey during/after Chem class:
![]()
--------->

***Definitely much shorter and....fine, I'll nice for once...a bit slimer...***
And then, there's me, A.L. I love to elaborate on others but definitely not myself. If anyone wishes to do so, go right ahead before Monday. But, here's me, the least interesting one, best portrayed by Ashley Spinelli.

Something a little less grumpy

** Take away the toque, the red dress, the striped tights, the boots...we have nothing alike.. sigh**
Alrighty, it seems like you guys all know us now. And this horrifying year-long nightmare is finally coming to an end. LOL. We miss you... not. Yup we're leaving now, goodbye. Before that, we wish you luck in the upcoming two chemistry tests with the aid of our blog. IF you really love us, leave us a comment below, but don't expect us to get back to you so soon because we're really busy people. I mean it. Okay,
さよなら!(sayonara, it means goodbye)!
P.S. here is our group photo, taken some years ago with our then-classmates, can you spot the four of us???
 School is going to be out soon, you can soon toss away all your chemistry notes...
School is going to be out soon, you can soon toss away all your chemistry notes...
 {Disclaimer (ONLY ONLY to our blog writers): the last section, aka the writers' biography section is all been done by a computer virus that has overwritten all the jokes I was intended to post. If anything offends you, I sincerely apologize for that. I've tried to remove them but it just doesn't seem to follow my command. Alright, I guess we'll just have to leave this alone in a corner of the internet.}
{Disclaimer (ONLY ONLY to our blog writers): the last section, aka the writers' biography section is all been done by a computer virus that has overwritten all the jokes I was intended to post. If anything offends you, I sincerely apologize for that. I've tried to remove them but it just doesn't seem to follow my command. Alright, I guess we'll just have to leave this alone in a corner of the internet.}






 Something a little less grumpy
Something a little less grumpy 
















 OR
OR




