Heating Curve
A: A solid, closely packed particles in an ordered manner, slow vibrating in fixed positions.
A→B: Gain of heat, KE1 ↑2, temperature ↑.
B: Particles still packed, similar to state A.
B→C: Changes from solid→liquid. Temperature does not change as heat energy is used to overcome the force of attraction (latent heat of fusion). Known as the melting point.
C: Liquid state.
C→D: Temperature ↑ KE ↑
D: Still a liquid, molecules begin to move more freely, starts to become a gas.
D→E: Changes from liquid → gas. Temperature does not change as heat is used to overcome the force of attraction. Known as the boiling point.
E: Gas state
F: As temperature continues to ↑, molecules vibrate faster and KE ↑.
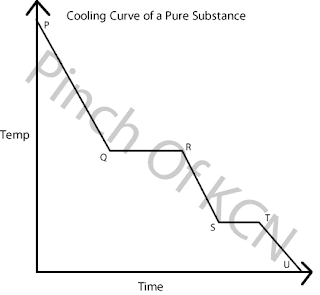
Cooling Curve
P: Gas state. High levels of vibration and KE.
P→Q: Temperature ↓3KE ↓.
Q: Still a gas, molecules begin to form bonds to create liquid.
Q→R: Condensation continues, changes from gas→liquid. Same temperature (latent heat of vaporization).Known as the boiling point.
R: Liquid state
R→S: KE↓ Temperature↓ Molecules lose energy and move closer.
S: Liquid state, beginning to become a solid.
S→T: Changes from liquid→solid. Molecules become ordered into a neat pattern. Known as the freezing point. Temperature remains the same (latent heat of fusion)
T: Solid state.
T→U: Temperature↓
U: Room temperature
Separation Techniques
The basis of separation is founded on different components have different properties, or the magnitude of these properties. Examples of properties include: density, reactivity, volatility, magnetism, solubility, polarity, etc.
Strategies of separation use processes that discriminate between these properties. Strategies include: filtration, flotation, crystallization, extraction, distillation, chromotography, and so on. Below is a chart classifying common methods to separate components.
Type of Matter |
Solid |
Liquid |
Gas |
Solid |
Gravity Separation |
Crystallization |
Adsoprtion |
Liquid |
Filtration |
Chromatography Distillation |
Centrifugation |
Gas |
Absorption |
Demister (tool) |
Adsorption |
1: Kinetic Energy
2: Increases
3: Decreases



No comments:
Post a Comment