1. Non-Polar Covalent bonds are formed when the difference between the values is less than 0.5
2. Covalent bonds are formed when the difference is between 0.5 and 1.8
3. Ionic bonds are formed when the difference is over 1.8
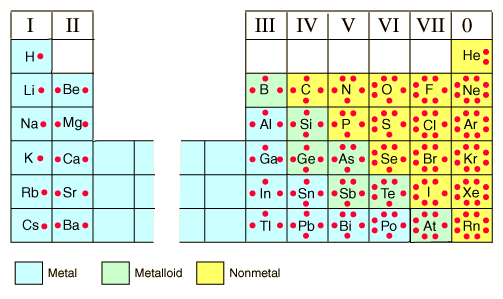
How to draw the structure of atoms and bonds will also be on the test, so scrutinize carefully. The below chart show the valence electrons of some elements.
You'll notice that the transition metals are omitted; that's because we won't be tested much on those. Also they vary and it's above our level, just like predicting the structure of certain bonds. If you're asked to draw one and not sure if it has a single, double, or triple bond, use your best guess. Try to make it symmetrical. You'll want to try and make all atoms have 8 electrons surrounding it. This goes for both covalent and ionic bonds. How are they different? See below.
Covalent bonds are like this.
A line represents a single bond of two electrons.
Ionic bonds are like this.
Put brackets around it and the charge on the outside.
YES. THIS UNIT IS THIS EASY SO FAR. DON'T FAIL EVEN IF MS CHEN IS NOT HERE. OK?OK.




No comments:
Post a Comment