Chemical Bonding Part 1
First, I would like to apologize to everyone to came to this blog for "My Chemical Romance", this blog is strictly about 2 types of chemical bonding not crazy death rock music...
Correct Wrong
Tip: Positive atom joins with Negative atom, usually the combining of Metals and Non-metals.
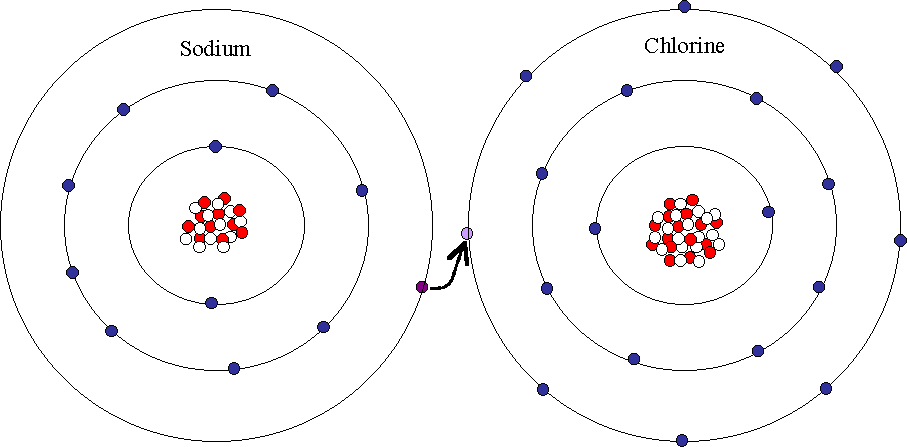
If you have forgotten about which elements have positive or negative charges, go back 2 posts with an EPIC period table. Atoms want to gain or lose electrons in their outer ring so it will have a full ring. Usually the Metals will lose/give their electrons to the Non-metals because they have a strong force and they need the electrons from Metals. Separate a Ionic Bond you say? Impossible you say? Not impossible! You can actually separate these bonds by using very high melting point. In the picture below, you can see Sodium will give the extra 1 electron to Chlorine = both atoms have a full outer ring!!!
Eg.
(Non-Polar) Covalent Bonding
Tip: Negative atom joins with Negative atom, the combining of Non-metals and Non-metals...
Usually it is just called Covalent Bonding(don't need the non-polar). Metal does not participate in Covalent Bonding. Same concept as Ionic Bonding, 2 elements sharing their electrons this time to yield a full ring of electrons. Some of you may wonder, how do these particles hold each other? Obviously not with glue, instead it uses intramolucular forces to hold the molecule. But what puts them together? Intermolecular forces. How do I remember them? Here are some bad examples, Intramolecular, think of Intra Muscular holding the molecule together RAWR. For Intermolecular, think of what you need to get a job, AN INTERVIEW. SEE WHAT I DID THERE?
Eg.
Here a video for the illiterate kids...







No comments:
Post a Comment