Atomic Structure
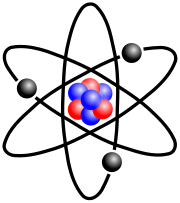 Now as we all know, everything in the observable universe is made up of teeny-tiny round things, called atoms. What makes up atoms you ask? Well, mostly nothing. I'm not even kidding when I say it's 99.9999999999999% empty space. Shocking, isn't it? To know that everything you see could probably fit inside your pocket if completely compressed. Well, in chemistry, we don't care about that, so let's move on to the "important stuff".
Now as we all know, everything in the observable universe is made up of teeny-tiny round things, called atoms. What makes up atoms you ask? Well, mostly nothing. I'm not even kidding when I say it's 99.9999999999999% empty space. Shocking, isn't it? To know that everything you see could probably fit inside your pocket if completely compressed. Well, in chemistry, we don't care about that, so let's move on to the "important stuff".Most of the mass of an atom lies in its nucleus, where the neutrons and protons are snuggled cozily together. Outside this central mass floats an electron cloud. Electrons orbit the nucleus in this cloud. In fact, they do this in such an odd way, it is impossible to determine the location of an electron at any given moment. This is quantum theory, and if you want to find out more, read the newspaper article I've yet to write. (Basically they "teleport" around in the electron cloud.)
Now that you're familiar with these subatomic particles, let's see what the purpose of their non-sentient existence is.
Electrons
 The particles most easily gained and removed are called electrons, which are negatively charged. Along with protons, these particles determine the charge of an atom. Actually, once an atom has lost or gained electrons, it is called an "ion."
The particles most easily gained and removed are called electrons, which are negatively charged. Along with protons, these particles determine the charge of an atom. Actually, once an atom has lost or gained electrons, it is called an "ion."Typically, metals give off electrons and non-metals take electrons. You can imagine the metals as boys because they always give — I'm going to stop before I make an outrageously sexist comment. Moving on!
Negatively charged ions are called
Neutrons
Neutrons (a particle without a charge), along with protons, give an atom its weight. Now, atoms, even those of the same element, don't all have the same weight. If your typical Carbon-12 atom, say, gained a couple of neutrons, it would become Carbon-14; the same element, just a bit heavier. There's nothing wrong with being Carbon-14, of course, but it would put that particular atom in a world where Carbon-12 reigns.
 Why do atoms have different weights? The simple answer is because some are more stable. With all those neutrons and protons fighting for space, a little nudge will send a neutron flying away. Atoms with different weights from what is most common for their element are called isotopes. A well-known example is Uranium-235, used for nuclear bombs due to their instability.
Why do atoms have different weights? The simple answer is because some are more stable. With all those neutrons and protons fighting for space, a little nudge will send a neutron flying away. Atoms with different weights from what is most common for their element are called isotopes. A well-known example is Uranium-235, used for nuclear bombs due to their instability.Protons
What makes an element an element? The answer is protons (positively charged, you know, "pro"). A hydrogen atom has one proton; Carbon has 6, Nitrogen has 7. The atomic number of an element is also the number of protons it contains. What do you think happens when an atom gains or loses a protons? BAM, element change. This is alchemy, what those crazy bearded guys in ancient Greece and Egypt tried to do. (Well they just wanted to make gold, actually). Little did they know, alchemy is impossible with chemical processes. And we're honestly wondering why physicists laugh at us?
Conclusion
Well that wraps it up for this blog post! If you have any questions, ask the teacher. Seriously, learn to ask questions instead of sitting there and watching your grade point average steadily drop. We'll be back whenever, with whatever, written by whomever.
What's that? You want a joke? No.

No comments:
Post a Comment